Research

Environmentally benign oxidation
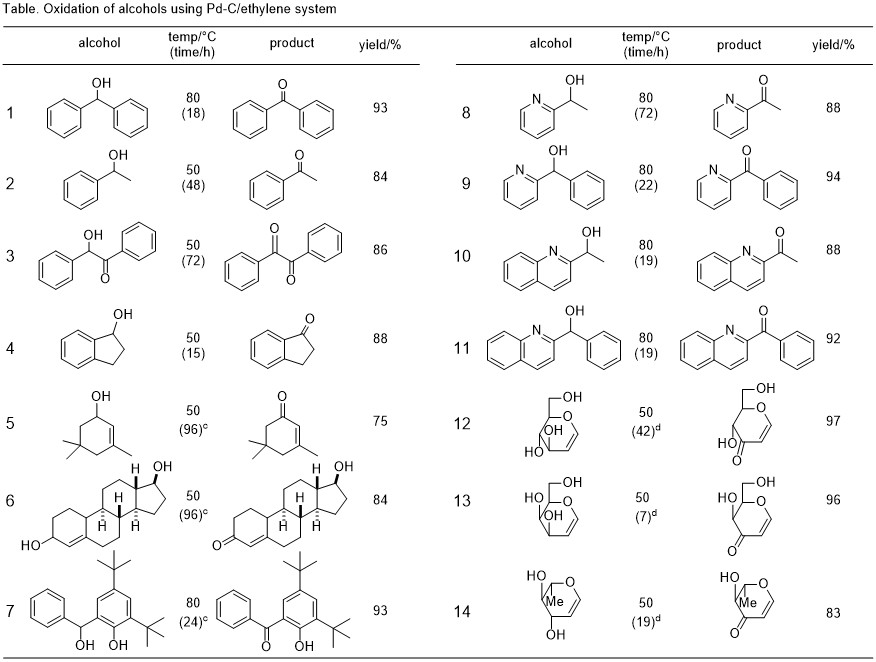
Pd/C-ethylene by the oxidation reaction of alcohol8
Conversion of alcohols to aldehydes or ketones is one of the most important reactions in organic synthesis. However, in the conventional oxidation reaction, a stoichiometric amount of chromic acid or manganese dioxide has been used. Methods of using these oxidizing agents are not desirable from the viewpoint of toxicity and treatment of waste. In our group, we worked on the development of the oxidation reaction of alcohol that does not put a load on the environment. Then, we found a hydrogen transfer reaction by Pd-C/ethylene system, and we achieved an efficient conversion of the secondary benzyl alcohol and allyl alcohol to the corresponding carbonyl compounds.
Pd-C catalyst used in this reaction is readily recovered can be reused, since the co-product is only ethane gas, this oxidation reaction is extremely friendly to environment.
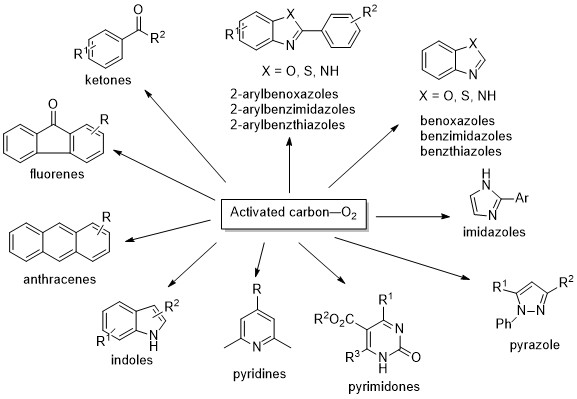
Oxidation Using Activated Carbon and Molecular Oxygen System9
The activated carbon—molecular oxygen system is efficient not only for the oxidation of benzylic and allylic alcohols to carbonyl compounds, but also for direct carbonylation at the benzylic position. A variety of heteroaromatic and aromatic compounds, including substituted pyridines, pyrazoles, benzoxazoles, benzimidazoles, benzothiazoles, 2-substituted imidazoles, indoles, pyrimidin-2(1H)-ones, and anthracenes, have been prepared by oxidative aromatization using the activated carbon—molecular oxygen system. To clarify the reaction mechanism in order to obtain information of the role of activated carbon, we examined oxidative aromatization using more than ten kinds of activated carbon having different surface areas, micropore volumes, and oxygen-containing functional groups. We revealed that the multiplier effect of surface area and the content of the oxygen functional group evolving as CO in micropores and mesopores played a very important role in promoting an efficient reaction.
The compounds synthesized by activated carbon-oxygen
References(review)
9) a) Y. Kawashita, N. Nakamichi, H. Kawabata, and M. Hayashi, Org. Lett., 5, 3713 (2003). b)M. Hayashi, Chem. Rec., 8, 252 (2008).
