Research

Asymmetric catalysis
Catalytic asymmetric reaction using novel chiral Schiff base ligand-metal complexes
1. Catalytic asymmetric silylcyanation of aldehydes
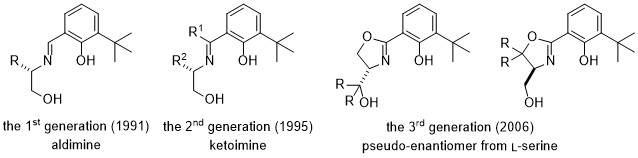
In 1991, we firstly reported the highly enantioselective silylcyanation of aldehydes using novel chiral Schiff base - titaniumu complexes (the 1st generation).1
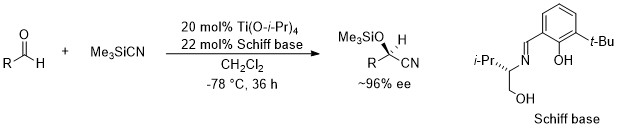
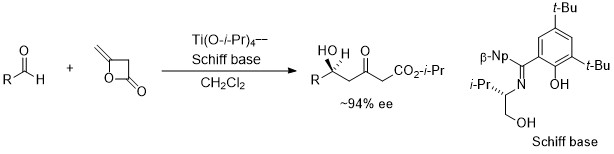
2. Enantioselective addition of diketene to aldehydes.
Highly enantioselective addition of diketene to aldehydes were achieved to provide optically active 5-hydroxy-3-ketoesters.2 By changing from aldimine to ketoimine, high chemical yield than aldimines, has achieved a high asymmetric yield (the 2nd generation).
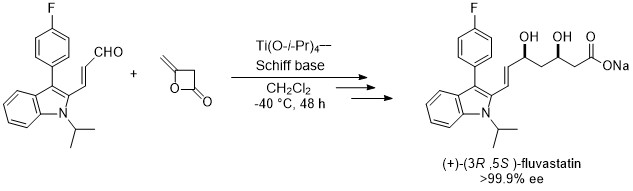
The short-step synthesis of the HMG-CoA reducing-enzyme inhibitor was successfully achieved with an optical purity of > 99.9% ee in three steps by using the enantioselective addition of diketene to aldehyde. This is the first example of asymmetric synthesis fluvastatin. Fluvastatin analogues were also synthesized by the same strategy.3
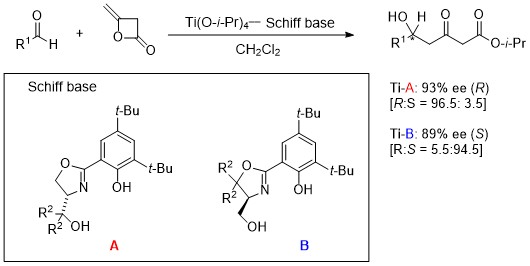
Reverse phenomenon of enantioselectivity5
To obtaining both enantiomers in high enantiomeric excess is an attractive and important challenge, because it is often the case that each enantiomer exhibits different bioactivity. Our new approach achieved complete reversal of enantioselection in the enantioselective addition of diketene to aldehydes affording optically active 5-hydroxy-3-keto esters in high optical yield using Schiff bases that contain an oxazoline moiety derived from L-serine (the 3rd generation). This means both enantiomers were obtained from single chiral source.
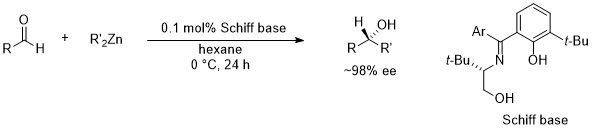
3. Catalytic enantioselective alkylation of aldehydes4b
Chiral Schiff base-zinc complexes have been developed for enantioselective alkylation of aldehydes. In this case, chiral zinc complexes those are generated from chiral Schiff base and the dialkylzincs are working the catalyst.
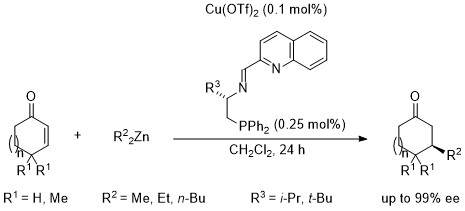
4. Catalytic asymmetric 1,4-addition of dialkylzincs to enones
Recently, we developed novel type of N,N,P Schiff base ligands in place of O,N,O Schiff base ligands. Enantioselective copper-catalyzed 1,4-addition of dialkylzincs to enones proceeded in the presence of 0.1 mol% of Cu(OTf)2 and 0.25 mol% of an N,N,P-ligand containing a quinoline moiety to afford the corresponding conjugated adducts in 99% ee. The intermediate zinc enolates were trapped with substituted allyl iodides to give disubstituted ketones with high diastereoselectivity and enantioselectivity.6
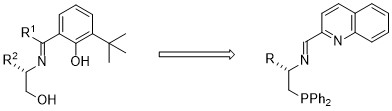
Design of novel N,N,P-tridentate ligand
Enantioselective 1,4-addition of dialkylzincs to enones
5. Catalytic asymmetric allylic oxidation
New N,N-bidentate Schiff base ligands containing the 2-quinolyl moiety proved to be effective in the copper (I)-catalyzed asymmetric allylic oxidation of various cyclic olefins with tert-butyl perbenzoate. Asymmetric desymmetrization of allylic oxidation of 4,5-epoxycyclo-hex-1-ene took place in the presence of 2.5 mol% of Cu(CH3CN)4PF6 and 3 mol% of the designed N,N-bidentate ligands to afford 3-benzoyloxy-4,5-epoxycyclohex-1-ene in 84% ee, which was increased up to >99% ee after recrystallization of the nitrobenzoyloxy derivative.7

By using the above mentioned asymmetric allylic oxidation reaction, we achieved the catalytic asymmetric synthesis of oseltamivir phosphate (Tamiflu).7c
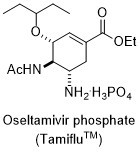
One for the purpose of the organic synthesis is the synthesis of physiological activity compound such as the pharmaceuticals and natural products. However, here I would like to emphasize that we did not have a plan to synthesize fluvastatin and Tamiflu from the beginning. After performing enantioselective addition reaction of diketene to the aldehyde with a chiral titanium catalyst, we decided to synthesize statins. In the case of the synthesis of Tamiful was also based on the discovery of chiral copper catalyst effective for asymmetric allylation. Thus, generality of our approach is exclusively high, can be used for the ultimate short-step synthesis of all statins and the synthesis of Tamiflu derivatives is also possible. If we would set the target compound from the beginning, if you do not go to the synthesis planned ideal strategy, we would select the next best thing. Repeat of this would lead us far from ideal strategy. We always aim to advance to the "simplicity, clarity and logic".
In our group we have achieved several catalytic asymmetric carbon-carbon bond forming reactions using originally designed chiral Schiff base -- metal complexes. as catalysts such as titanium and zinc complexes. Specifically, we firstly reported catalytic asymmetric silylcyanation of aldehydes catalyzed by chiral Schiff base -- titanium complexes. The chiral Schiff base -- zinc complexes were proved to be excellent catalyst of asymmetric alkylation of aldehydes using dialkylzincs. Furthermore, the enantioselective addition of diketene to aldehydes were also accomplished using chiral Schiff base -- titanium complexes. By utilizing the asymmetric reaction short-step synthesis of HMG-CoA reductase inhibitor was achieved.
References
2) a) M. Hayashi, T. Inoue, and N. Oguni, J. Chem. Soc., Chem. Commun., 341 (1994). b) M. Hayashi, T. Inoue, Y. Miyamoto, and N. Oguni, Tetrahedron, 50, 4385 (1994).
3) a) M. Hayashi, K. Yoshimoto, N. Hirata, K. Tanaka, N. Oguni, K. Harada, A. Matsushita, Y. Kawachi, and H. Sasaki, Isr. J. Chem., 41, 241-246 (2001). b) J. T.Zacharia, T. Tanaka and M. Hayashi, J. Org. Chem., 75, 7514 (2010).
4) a) M. Hayashi, K. Tanaka, and N. Oguni, Tetrahedron: Asymmetry, 6, 1833 (1995). b) T. Tanaka, Y. Sano, and M. Hayashi, Chem. Asian J., 3, 1465 (2008).
5) a) C. H. Chu, K. Morishita, T. Tanaka, and M. Hayashi, Tetrahedron: Asymmetry, 17, 2672―2677 (2006).b) T. Tanaka and M. Hayashi, Synthesis (review), 3361 (2008). c) 田中孝徳,林 昌彦,有機合成化学協会誌, 65, 969 (2007).
6) a) K. Kawamura, H. Fukuzawa, and M. Hayashi, Org. Lett., 10, 3509 (2008). b) K. Kawamura, H. Fukuzawa, and M. Hayashi, Bull. Chem. Soc. Jpn., 84, 640 (2011). c) Y. Ebisu, K. Kawamura, and M. Hayashi, Tetrahedron: Asymmetry, 959 (2012).
7) a) Q. Tan and M. Hayashi, Adv. Synth. Catal., 350, 2639 (2008). b) Q. Tan and M. Hayashi, Org. Lett., 11, 3314 (2009). c) T. Tanaka, Q. Tan, H. Kawakubo, and M. Hayashi,, J. Org. Chem., 76, 5477 (2011).
