
In the last century the global population quadrupled, while the world water demand increased sevenfold. The World Water Council estimates that by 2030, 3.9 billion people will live in regions characterized as “water scarce”. In addition to overall water shortage, poor water quality is near major crisis in many parts of the world which leads to several diseases. It is very crucial to protect existing fresh water resources and to develop new water resources in order to meet the world’s growing demand for clean water. This will require better water treatment technology.
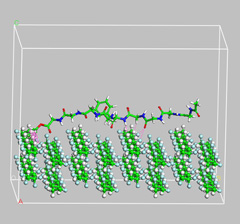 Fig. 1: Interaction of PVDF membrane with peptide molecule. |
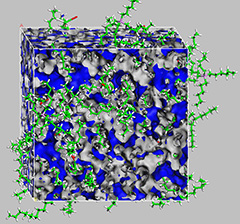 Fig. 2: Free volume for P66614-GLY ionic liquid pair. |
Membranes are favoured over many other technologies for water treatment because, in principle, they require no chemical additives or thermal inputs and they do not require regeneration of spent media. During the past two decades, reverse osmosis (RO) has become the main technology for the production of drinking and ultrapure water for the electronics and pharmaceutical industry. Sea water desalination and purification of brackish water are of particular interest considering the level of importance and urgency that providing enough drinking water for the growing World’s population. The heart of every RO system is the semipermeable membrane which permits passage of pure water while rejecting dispersed and dissolved solids, and both technological and economical aspects of the process are directly affected by its properties and performance.
At present several excellent polymer membranes are available however these polymer membrane which readily attract organic and biological species that quickly build up an additional filtration barrier that reduces the flux of permeate water through the membrane. The accumulation of foulant molecule on membrane surface is called as membrane fouling. Membrane fouling is costing much more as several steps needed to be taken to stop or reduce fouling. It includes: increasing the operating pressure by as much as 50% in some cases, interrupting production for cleaning the membranes by frequent chlorination, and eventually replacing the entire membrane elements sooner than desired. Mainly, the cost of energy required by the high-pressure pumps to remedy the fouling-induced flux decline often amounts to ca. 30% of the total operating expense and become the main factor that controls the economics of the water production by RO processes.
As a consequence, there is a fast growing need for effective prevention of membrane fouling in RO processes, and among the methods under consideration, surface modification of existing, commercially available membranes by antifouling coatings has attracted considerable research attention. In our group we are using both experimental and computational approaches in order to understand membrane fouling.
In our research, we mainly focused on interaction of polymer membrane with foulant. Computational studies has always been very effective from drug design to membrane design. In our research we are using molecular dynamics simulation to understand interaction of membrane with organic foulant. The membrane such as Polyvinylidine difluoride (PVDF), Cellulose Acetate (CA), polyether sulfone (PES) is used in our studies and its interaction with peptide (Fig. 1), sodium alginate and other foulant is carried out. Not much research has been devoted to understand the phenomenon of membrane fouling and such studies will be helpful to design new membrane with improved antifouling properties. Finally, the combined approach of experimental and computational methods will help in such a way that membrane fouling is minimised. It will help to increase the membrane lifespan and reduce the operational cost for water treatment.
In addition we are interested in preparation of ionic liquid for CO2 gas separation. Ionic liquids are slats with a melting point below 100 C and can be made from different combinations of cation and anion such as tetrabutyl phosphonium and glycine (Fig. 2). These ILs are used in dye-sensitized solar cells and also make promising extraction and separation of gases such as CO2 and N2. Knowledge of thermophysical properties are essential for design of chemical plants and processes. These measurement however need enormous investment in time and resources. In this framework, molecular dynamics is very important tool for both understanding of the structure-property relationships and rational design of new ILs.