Research
Research background
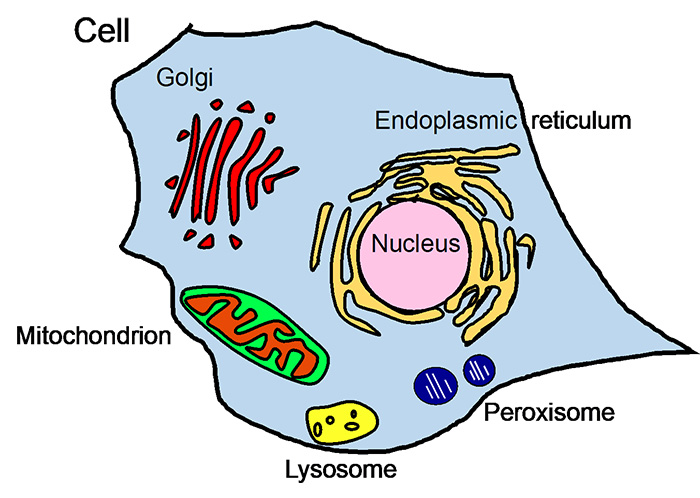
Fig. 1 Organelles
Mammalian cells have organelles such as nucleus, endoplasmic reticulum (ER), Golgi apparatus, mitochondria, lysosomes, peroxisomes (Fig. 1).
Approximately 1/3 of all proteins are synthesized in the endoplasmic reticulum. Examples include hormones (e.g., insulin) and membrane proteins (e.g., adrenergic receptors) that are produced in a secretory pathway.
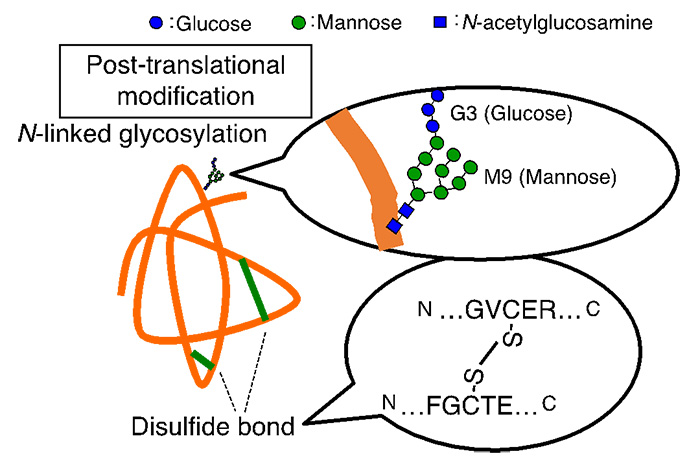
Fig. 2 Post-translational modification of proteins in the ER
Newly synthesized proteins in the endoplasmic reticulum undergo post-translational modifications such as N-glycosylation and disulfide bond formation (Figure 2). N-glycans are composed of 14 hydrophilic monosaccharides (3 glucose, 9 mannose, etc.). It is thought that the addition of N-glycans improves the solubility of proteins and aids in their three-dimensional structure formation. Disulfide bonds reinforce and stabilize the three-dimensional structure of proteins.
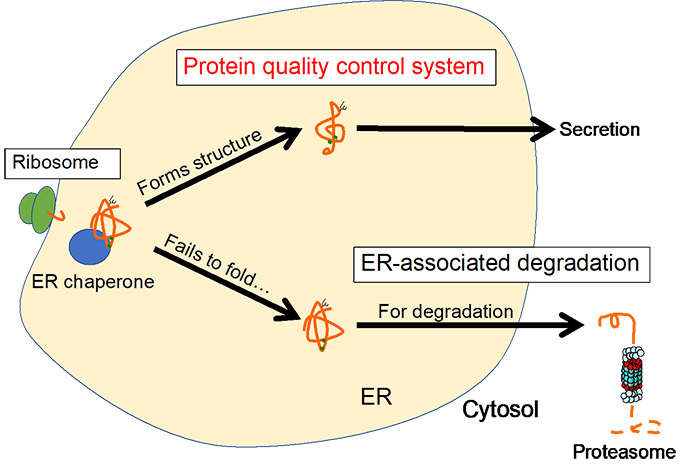
Fig. 3 Protein quality control in the ER
In addition to these post-translational modifications, molecular chaperones (ER chaperones) promote folding of proteins, enabling them to acquire their correct three-dimensional structure (Figure 3). Proteins that have acquired a three-dimensional structure proceed to the next secretory pathway, in the Golgi apparatus. Proteins that cannot fold properly are transported back into the cytosol through the retrotranslocation complex with simultaneous ubiquitination, extracted by AAA-ATPase and degraded by the proteasome in the cytosol. This sequential process is referred to as ER-associated degradation (ERAD).
Thus, the ER is equipped with a protein quality control mechanism that directs proteins that have formed their structure to the secretory pathway and those that cannot form their structure to the degradation pathway.
In our laboratory, we are conducting research mainly on functional analysis of genes using human cultured cells to elucidate these quality control mechanisms. This is linked to human diseases such as Alzheimer's disease, Parkinson's disease, diabetes, rheumatism, cystic fibrosis, Huntington's disease, and muscular dystrophy. Our research will be useful in the future in developing new therapeutic and preventive strategies.
To learn more about protein quality control mechanisms in the ER, go to the next page.
ER protein quality control system and N-glycan
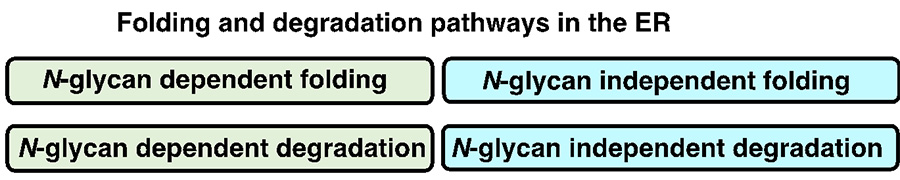
Fig. 4 Classification of protein folding and degradation pathways in the ER
N-glycans are said to modify about 80% of proteins that pass through the endoplasmic reticulum, improving the hydrophilicity of proteins and assisting in proper protein conformation. It is also known that the presence or absence of N-glycans significantly affects the folding and degradation processes of glycoproteins in the ER. There are N-glycan-dependent and N-glycan-independent pathways for protein folding and degradation (Fig. 4), and thus the presence or absence of N-glycans has a significant effect on the protein folding and degradation in the ER.
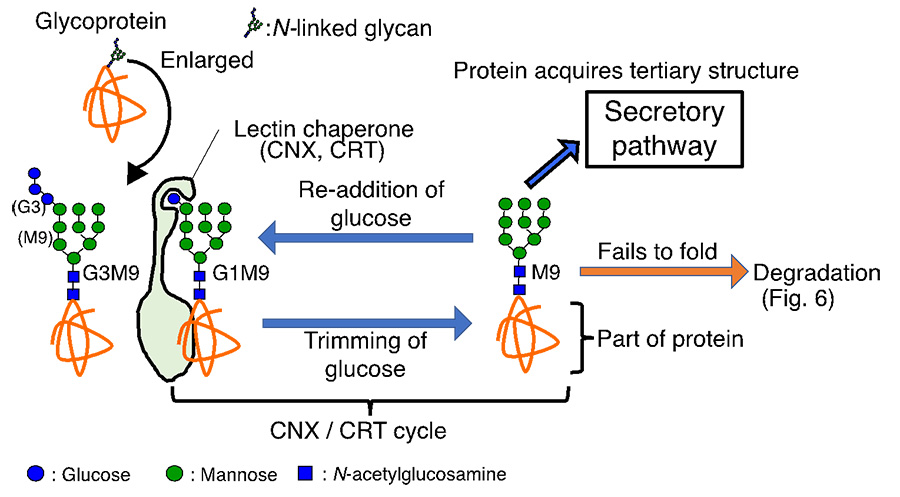
Fig. 5 CNX/CRT cycle promotes folding of glycoprotein
Here, the N-glycan-dependent folding and degradation pathways are described in detail. The N-glycan is composed of three glucoses, nine mannoses, and two N-acetylglucosamines (Abbreviated as G3M9 because there are 3 glucoses and 9 mannoses) (Fig. 5). When two glucoses are trimmed to form G1M9, the lectin chaperones Calnexine (CNX) and Calreticulin (CRT) recognize the glycan structure and promote folding of the glycoprotein. Then, after one remaining glucose is trimmed, correctly folded glycoproteins proceed to the next secretory pathway. If the proteins cannot fold, glucose is re-added to the glycoprotein, resulting in G1M9. Then it is recognized by CNX and CRT again to facilitate protein folding. This glycoprotein folding cycle is called the CNX/CRT cycle. Thus, an elaborate glycoprotein folding system dependent on the addition and removal of glucose is at work in the ER. If proteins still fail to form their structures, they proceed to the degradation process (Fig. 6).
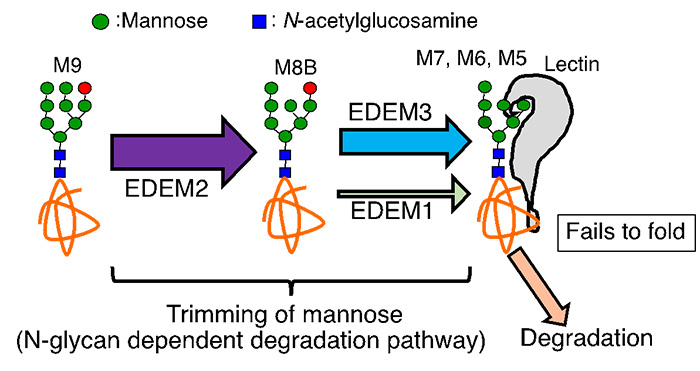
Fig. 6 Mannose trimming of N-glycan in the ER
When a protein containing M9 glycan cannot fold properly, one mannose is trimmed by EDEM2. Furthermore, mannose trimming from M8 to M7 is performed mainly by EDEM3 and to a lesser extent by EDEM1. As a result, mannose, shown as a red circle in the Fig. 6, is trimmed, allowing lectin degradation factors to recognize the glycans, leading to degradation of the glycoprotein (Fig. 6).
Taken together with Figure 5, this indicates that a glycoprotein that tried, but failed, to acquire the correct three-dimensional structure is destined for degradation by a two-step mannose trimming. We have proposed and established a novel molecular mechanism for mannose trimming in the ER (Ninagawa et al., 2014 JCB). This has allowed us to make great strides in understanding one of the biggest problems in ERAD: how the proteins to be degraded are selected.
If you are interested in hearing about/doing any of studies related above, we look forward to hearing from you. We would be happy to discuss our research with a wide range of people.