1. Supramolecular gelator (low-molecular-weight gelator).
Although gels are usually made from polymers such as gelatin or agarose, they can also be created from low-molecular-weight gelators, also called supramolecular gelators. These small molecules spontaneously self-assemble into nanofibers or nanosheets, and in doing so, they act like polymers to solidify solvents into gels. We have developed various types of supramolecular gelators and succeeded in turning water, organic solvents, and even ionic liquids into gels at low concentrations. Our current goal is to create functional gels using supramolecular gelators, including highly conductive ionogels, hydrogels with anticancer activity, and gels that can be used to carry out chemical reactions.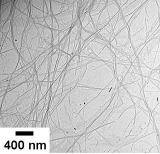 
We recently synthesized
・Supramolecular gelator that can harden a wide variety of water, organic
solvents and ionic liquids.
・Supramolecular gelator that can kill cancer cells.
・Supramolecular gelators that can harden ionic liquids (conductive ionogel).
These gels have potential to be used in pharmaceutical and medical fields and also in electronic device.
W. K. Restu et al., Colloid Polym. Sci. 295, 1109-1116 (2017).
Y. Nishida el al., Angew. Chem. Int. Ed. 56, 9410-9414 (2017).
W. K. Restu el al., Colloid Polym. Sci. 295, 1109-1116 (2017).
Y. Eguchi et al., Chem. Commun., 53, 5802-5805 (2017).
T. Kataoka et al. ACS Appl Mater Interf. 7, 23346–23352 (2015).
A. Tanaka et al., J. Am. Chem. Soc. 137, 770-775 (2015).
Y. Ishioka et al., Soft Matter 10, 965-971 (2014). 
N. Minakuchi et al., Langmuir 28, 9259−9266 (2012).
D. Koda et al., Chem. Commun., 46, 979-981 (2010).
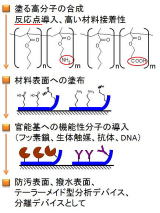
2. Surface functionalization of polymer using polymer segregation.
Material surfaces play particularly important roles in both our daily lives and in industry. Phenomena such as touching, wetting, impact, and confinement are all closely related to surfaces. Our research focuses on developing methods to control the physical and chemical properties of these surfaces. To achieve this, we design and synthesize functional polymers, then coat material surfaces with them to adjust their characteristics. Our goals include creating non-fouling surfaces, catalytically active surfaces, and surfaces that can specifically recognize target molecules for use in separation technologies and chemical analysis.
R. Sakai et al., RSC Adv. 9, 4621-4625 (2019)..
M. Hara et al., Polymer J. 51, 489-499 (2019)).
K. Nishimori et al., Langmuir 34, 6396–6404 (2018).
R. Hiraoka et al., Chem. Comm., 51, 17447-17450 (2015).
S. Shiota et al., Langmuir 31, 8824-8829 (2015).
S. Yamamoto et al. Langmuir 31, 125-131 (2015).
A. Shimomura et al. Langmuir, 29, 932-938 (2013).
3. Novel functions derived from self-assembly (aggregation) of synthetic
molecules.
Based on our studies of supramolecular gelators, we have found that when many molecules gather and self-assemble, they can show completely new functions. In particular, we discovered new enzyme inhibitors and unique interactions between peptides, which may lead to the development of new drugs. Through this work, we aim to show the world that even relatively simple synthetic molecules, when allowed to self-assemble, can display entirely new pharmacological activities.

4. Design and synthesis of functional surfactants that can exceed conventional detergents.
Surfactants are widely used as detergents. We try to develop surfactants
that have novel functions beyond "washing detergents". For example,
the figure shows the surfactants that have "separation ability".
We developed a surfactant having oligo DNA, which can separate complemtary
DNA, and a surfactant that can recognize proteins to be introduce on a
membrane surface. These surfactants successfully separate and purify target
DNA, RNA and proteins that were produced by enzymatic reactions and recombinant
E. coli. We would like to expand these achievements to the fundamental techniques
required for the future industry and biotechnology. 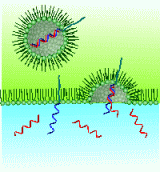
H. Iguchi, et al., Sci. Rep.7, 39937 (2017).
T. Maruyama et al., Chem. Commun., 52, 12376-12379 (2016).
C. Higashi et al., RSC Adv., 6, 88244-88247 (2016).
T. Maruyama et al, ChemNanoMat, 2, 461-465 (2016).
T. Kato et al, RSC Adv. 4, 57899-57902 (2014).
T. Honjo et al., Anal Biochem., 434, 269-274 (2013).
T. Maruyama et al. RSC Adv., 2, 125–127 (2012).
T. Maruyama et al., Chem. Commun., 4450-4452 (2007).
5. Design and formation of microstructures prepared by electrospinning
and electrospraying 
The electrospinning/electrospraying technique has a great potential for
preparation of nano- and micro-fibers because of the simplicity of the
apparatus, its high productivity and its easy setup. During the past decades,
the electrospinnning technique has attracted more and more attention as
novel techniques of nanotechnology and microtechnology. We employed the
electrospinning/electrospraying technique to prepare polymeric and inorganic
microcapsules, and short fibers functionalized with biomolecules.
T. Matsuura et al.,Colloid Polym. Sci. 296, 239-244 (2018). .
T. Matsuura et al.,Colloid Polym. Sci. 295, 1251-1256 (2017); Colloids
Surf. A 526, 64-69 (2017).
Y. Funasaki et al. Colloid Polym. Sci. 292, 3049-3053 (2014).
A. Yunoki et al., ACS Appl. Mater. Interf. 6, 11973–11979 (2014).
T. Maruyama et al. RSC Adv., 2, 11672–11674 (2012).
Y. Fukui et al. Colloids Surf. A, 370, 28-34 (2010).
6. Recycling of precious and rare metal ions using protein-rich biomass.
Recently, the prices of precious metals and rare metals fluctuate violently
because of drastic increases of an industrial demand and a speculative
factor. These metals may be contained in industrial wastes. Effective use
of precious metals and the rare metals can be achieved by separating and
collecting them from such a waste solution. There are already several methods
for recycling these metals but these methods have some drawbacks to a varying
degree. On the other hand, metal ions in vivo form complexes with various
biomolecules (proteins and saccharides) and they exhibit unique functions.
This lets us speculate that it is possible to selectively separate and
collect the metal ions using biomolecules. In this study, we tried to develop
the technique for recycling precious metals from industrial wastes by using
protein-rich biomass (egg-shell membrane).
T. Maruyama, et al., Process Biochem. 49, 850-857 (2014).
T. Maruyama et al., Environ. Sci. Technol. 41, 1359-1364 (2007).
T. Maruyama, et al., J. Colloid Interf. Sci. 447, 254–257 (2015).
|









