6th Pacific Regional Wood Anatomy
Conference 2005 Kyoto Japan
Abstracts p.64-65, 2005
Visualization of a host reaction in oak stems infected with a
wilt pathogen, Raffaelea quercivora,
by magnetic resonance imaging
Keiko Kuroda,1 Yu Ichihara,2 Yoshiyuki
Kanbara,3 Takashi Inoue,4 and Akira Ogawa4
1) Kansai Research Center,
Forestry and Forest Products Research Institute, Momoyama, Fushimi,
Kyoto 612-0855, Japan.
Email: keiko@affrc.go.jp; HP:
http://cse.ffpri.affrc.go.jp/keiko/hp/kuroda-e.html
2) Tohoku Research Center, Forestry and Forest Products Research
Institute, Morioka 020-0123, Japan.
3) High Field Magnetic Resonance Imaging Research Institute,
Iwate Medical University, Takizawa-Mura, Iwate 020-0173, Japan.
4) Department of Neurosurgery, Iwate Medical University School
of Medicine, Morioka 020-8505, Japan.
Numerous
deciduous oaks, Quercus serrata
and Q. crispula, are killed
every year in Japan by a fungus, Raffaelea
quercivora. This fungus enters the sapwood vectored by an
ambrosia beetle, Platypus quercivorus,
which makes mass attacks on healthy oak trees. Anatomical
investigations of infected trees revealed that widespread discoloration
(wound heartwood) had occurred in sapwood (Kuroda 2001). The
present report discusses the mechanism of wilting based on the
non-invasive observation of infected trees by magnetic resonance (MR)
imaging. This technique is helpful for detecting the water
distribution and lesions caused by pathogenic microorganisms in living
trees (Kuroda 2005).
Q. crispula
saplings (diameter: 20-40mm, height: ca. 160cm) planted in pots were
inoculated with R. quercivora through one hole or four holes (diameter:
2.5mm) made on the lower stems. Ten inoculated saplings and five
controls were analyzed using an MR imaging system (Signa VH/i 3.0 T;
General Electric Medical Systems) at 7- or 10-day intervals for 42
days. Then, saplings were cut, and the bases were soaked in
aqueous acid fuchsin. Proton density MR images of healthy tree
stems showed cambium and functional vessels as high intensity. In
the MR images of the stems inoculated with the pathogen, a darker area
around the inoculated holes appeared and was observed to gradually
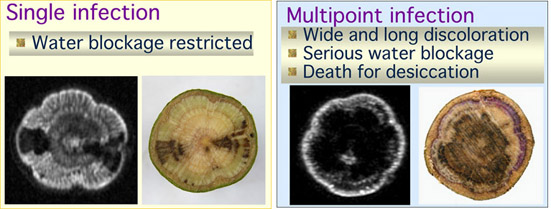
enlarge. In the specimens inoculated from one hole, the darker
areas were observed to elongate about 50mm for six weeks.
Anatomical observations after MR imaging revealed that the darker area
in the MR images coincided with the dysfunctional area that had not
been dyed with acid fuchsin. Most of the saplings inoculated with
the pathogen through 4 holes started to show leaf discoloration 20 days
after inoculation. Proton density MR images indicated that, prior
to leaf discoloration, the dehydrated area had swiftly enlarged
horizontally almost covering the stem cross section and longitudinally
more than 200mm. T1-weighted MR images, which are used to detect
tissues rich in fat or protein, showed the area of the discolored xylem
with fungal distribution as whitish. MR and optical images
demonstrated that the sap flow to the shoots had been blocked at the
discolored xylem that had formed as a defense reaction of parenchyma
cells to the fungal activity. Thirty days after inoculation, the
MR images showed the accumulation of water in the xylem below the
inoculation holes and desiccation above the holes. Xylem sap
oozed from the inoculation holes showing that the root is still
functional after the sap at the lower stem had stopped ascending.
These results demonstrated that the swift and wide distribution of the
pathogen in sapwood was enhanced by the multipoint infection and that
complete cut-off of the water supply to the shoots induced wilt symptom
in oak trees.
Return to Top Page
