Proceedings of the IUFRO Working Party 7.02.02 Shoot and Foliage Diseases, Meeting at Hyytiala, Finland, 17-22 June, 2001. Finnish Forest Research Institute, Research Papers 829:17-23, 2002
The mechanism of tracheid cavitation in trees infected with
wilt
diseases
Keiko Kuroda
Kansai Research Center, Forestry and Forest Products Research InstituteMomoyama, Fushimi, Kyoto 612-0855, Japan
Phone: (81) 75-611-1201; FAX: (81) 75-611-1207
E-mail: keiko@affrc.go.jp; http://cse.ffpri.affrc.go.jp/keiko/hp/kuroda.html
Abstract
In trees infected with pine wilt disease, extensive embolism occurs in tracheids. The enlargement of a cavitated and dysfunctional xylem induces a water deficit and mortality in trees. This phenomenon was observed in a wilt disease in oak trees caused by Raffaelea sp. To confirm an assumption that this wilt mechanism is fundamentally similar in several wilt diseases, inoculation experiments were conducted on larch and spruce trees. In Larix kaempferi trees inoculated with Ceratocystis lalicicola, acoustic emission from the trunks increased before the initiation of apex wilting. This indicates that abnormal dehydration from tracheids was occurring in the infected trees. In the trees that rapidly developed symptoms, xylem dysfunction had progressed widely. The leaves of Picea jezoensis were partially discolored about one month after inoculation with C. polonica. The fungal hyphae were elongating radially through the ray tissue. Before the symptoms were visible, areas that were cavitated and dysfunctional appeared in the xylem. When the outer annual rings became dysfunctional, the sap ascent decreased significantly, and the leaves began to discolor. The dysfunctional areas were much wider than the areas plugged with resin. The fungal activity promoted secondary metabolism in ray parenchyma cells and is responsible for the cavitation. A similar mechanism that promotes embolism and inhibits the refilling of water in pine wilt also occurs in the wilt diseases of larch and spruce trees.
Keywords: Pine wilt, embolism, sap ascent, Raffaelea, Picea, Larix, Ceratocystis
Introduction
Pine wilt is caused by the nematode Bursaphelenchus xylophilus and is one of the most serious tree diseases in Japan. From the end of 1980s, many oak forests on Honshu Island have been damaged by Raffaelea sp. Ceratocystis spp. are killing spruce and larch trees in northern Japan. All the pathogens of these diseases are vectored by beetles. The leaves of the trees become discolored about three weeks after inoculation with the pine wood nematode (Kuroda et al. 1988). Usually, healthy trees do not wilt very easily, even when the supply of water is stopped for a certain period. Why these pathogens can kill trees so effectively is a question that remains to be answered. Furthermore, the cause of the swift death of the apical and cambial cells of a huge tree does not yet have a theoretical explanation.
In healthy trees, xylem sap ascends spirally in pine trunks. In trees infected with pine wilt, dehydrated areas emerge in xylem prior to leaf discoloration (Kuroda et al. 1988). Such dysfunctional areas enlarge, and the tree wilts within one or two months. It is clear that some kind of a system rapidly excludes the xylem sap from the tracheids (Kuroda 1989, 1991). In the case of oak trees infected with Raffaelea sp., the discoloration of sapwood and blockage of water are well underway before the appearance of other symptoms of wilt in the summer months (Kuroda 2001, Kuroda and Yamada 1996, Takahata and Ikeda 2001).
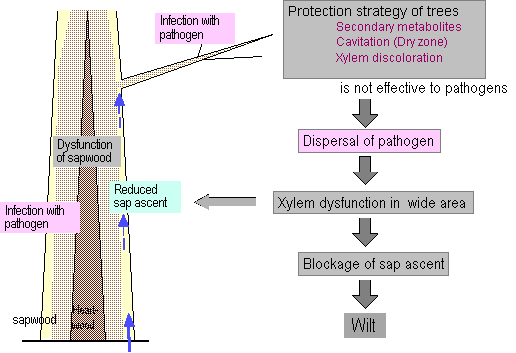
Fig. 1 Schematic illustration of wilting mechanisms hypothesized for the wilting disease of pine and oak trees.
The mechanism of the symptom development of wilt described here (Fig. 1) is based on research into the pine wilt and oak mortality caused by Raffaelea sp. When microorganisms attack a tree, a protection strategy is set in motion. A synthesis of secondary metabolites starts in the tree tissue. With this reaction, cavitation or discoloration occurs in the xylem. Tracheids and vessels become dysfunctional and are filled with air. Unfortunately, the secondary metabolites are ineffective in their efforts to protect the tree against the pine wood nematode and Raffaelea sp. As the pathogen is widely distributed, protection occurs in many places in a tree, and, therefore, xylem dysfunction is widely spread. The sap ascent is extensively reduced, and the tree dies because of a water deficit. I considered that these findings might be applicable to other wilt diseases. To check this possibility, wilt processes in larch and spruce trees were monitored after inoculation with the pathogens Ceratocystis lalicicola and C. polonica, respectively. Investigations were made with techniques involving Acoustic Emission (AE) to detect embolism and cavitation in tree trunks (Kuroda 1995, Kuroda and Kuroda 2000), a dye injected into tree trunks to trace the course of sap ascent, and anatomical analyses to check cytological reactions of host cells and hyphal distribution.
Materials and Methods
Japanese larch: On July 21, 1998, the pathogen of wilt disease Ceratocystis lalicicola Redfern et Minter* was inoculated on the lower trunks of seven-year-old Larix kaempferi Sarg. trees. The features of the inoculated and control trees are cited in Table 1. At the start of the experiment, AE transducers (140kHz) were attached to the lower trunks of the inoculated and control trees. This technique is useful for monitoring the embolism in living trees without cutting (Kuroda and Kuroda 2000). In the experiments on pine wilt, the extremely high rate of AEs was confirmed and reflected abnormal dehydration and dysfunction of the tracheids (Kuroda 1995). AE events were monitored until the harvest. The trees were checked for symptoms every week until the end of November.
Yezo spruce: On July 21, 1999, the pathogen Ceratocystis polonica (Siem.) C. Moreau** was inoculated into the trunks of seven-year-old Picea jezoensis (Sieb. et Zucc.) Carr. trees. Inoculated trees were harvested at one-week intervals. Table 2 shows the harvest dates and symptom development. AE transducers were attached onto the lower trunks prior to the inoculation. To visualize the water conduction, a dye solution was injected at the base of the trunks (1% aqueous acid-fuchsin) at harvest (Kuroda et al. 1988). The main stem was then dissected and processed for an anatomical analysis. Microscopic observations were made to check the reaction of the tree tissues to the fungal activities.
* Pathogen: Isolated by T. Yamaguchi from the blue-stained xylem of a L. kaempferi in the experimental forest at the Hokkaido Research Center, Forestry and Forest Products Research Institute.
** Pathogen: YCC- 115. Isolated by Y. Yamaoka from the gallery wall of P. jezoensis in the Tokyo University Forest in Hokkaido.
Results
and Discussion
Wilt
of Japanese larch
Two weeks after infection, the trees’ apices began to droop (Trees No.4 and 5 in Table 1, Fig. 2). Leaf yellowing and defoliation followed at three weeks after inoculation. These are characteristic symptoms of the disease. Figure 3 shows the frequencies of AEs detected around the time that the apices began to droop, which was the initial symptom. The typical pattern of AEs in healthy trees is indicated by the control data. In healthy trees, the AE pattern increases rapidly when the transpiration rates are high as a result of normal cavitation in the tracheids. There was no AE event at night. In inoculated trees, the frequency of AE was normal until August 24, and then it increased from August 25 just before the apices began to droop.
In pine wilt disease, it has been confirmed that cavitation occurs extensively in periods of high AE rates. The cavitated tracheids were not refilled with water. The increasing pattern of AE frequencies in larch trees resembles pine wilt, although the rates were lower. Therefore, the abnormal embolism and cavitation were assumed to have occurred in the periods of high AE rates in the trunks of infected larch trees. The shoots drooped because of a lack of water caused by the extensive embolism in the trunks.
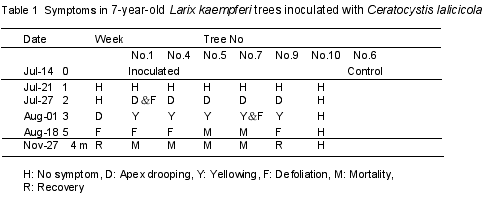

Fig. 2 Drooping symptom observed on Larix kaempferi inoculated with Ceratocystis lalicicola.
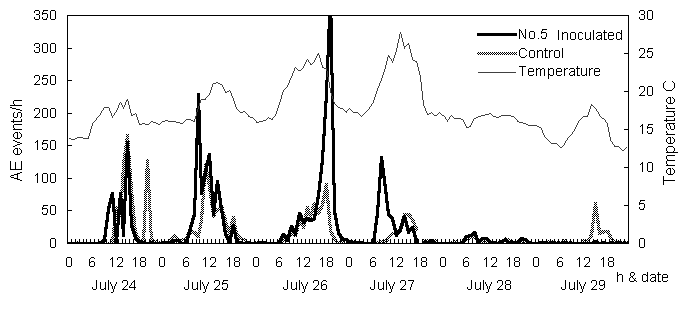
Fig. 3 High-frequency AEs detected from a trunk of L. kaempferi on July 26 and 27.
Wilt of Yezo spruce
The shoots of the spruce trees did not droop. Instead, their needles became discolored as an initial symptom (Fig. 4) about three weeks after the inoculation. In spruce trees, AE events did not increase as drastically, probably because the cavitation progressed gradually. As a result of dye absorption from the trunk bases, functioning tracheids were stained red. In the inoculated trees, white parts without staining were wider than the controls even before symptom development. Tracheids in those areas were filled with air and had become dysfunctional. Blockage of sap ascent was extensive by the start of leaf discoloration (Fig. 5A). This severe dysfunction caused water shortage and induced leaf discoloration.
Ray tissue in sapwood consists of living cells. Before symptom development, hyphae distributed through ray tissues, some resin canals, and a few tracheids. Vertical distribution was very narrow and limited. Radial elongation was deep and reached the depth of several annual rings. Ray parenchyma cells invaded by hyphae were necrotic. Ray parenchyma cells produced secondary metabolites as a reaction before necrosis, and the cell contents looked yellow. Cambial necrosis was just restricted to inoculated sites and was not serious. All these indicators showed that this fungus is not effective to kill cambium and that the cambial lesion in narrow area does not cause wilt. On the other hand, dysfunction and desiccation occurred in the xylem rapidly and widely. The activity of the fungus was effective to stop water conduction. It was significant that the dysfunctional area was much wider than the area of resin occlusion. Resin occlusion is not an essential factor for dysfunction, although it may also be effective to block sap ascent.
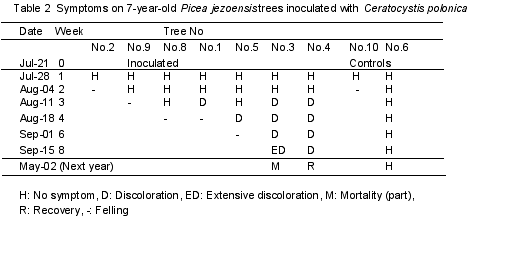

Fig. 4 Leaf discoloration observed on Picea jezoensis inoculated with Ceratocystis polonica.
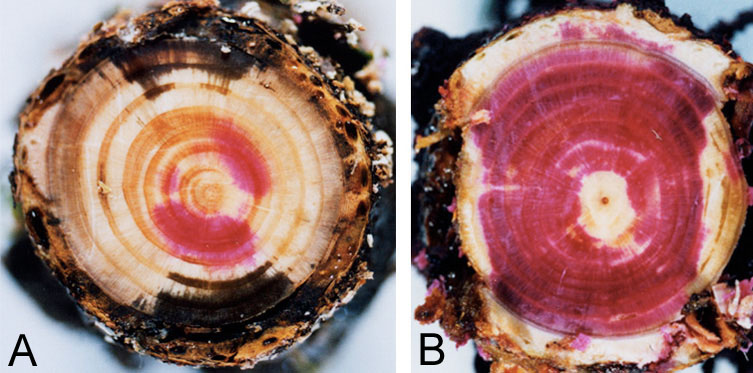
Fig. 5 Blockage of xylem sap ascent in P. jezoensis indicated by dye injection at the start of leaf discoloration (A) and normal water conduction in the control tree (B).
Conclusion
Distribution of two Ceratocystis species in larch and spruce trees was both slower and narrower than in the case of pine wood nematode; however, these fungi were effective to stop sap ascent. Complete stop of sap ascent is due to extensive cavitation (or embolism) of water conduits that cannot refill with water. This is the cause of wilt in these two wilting diseases. Ceratocystis spp. promoted secondary metabolism in parenchyma cells. As assumed in the case of pine wilt, substances with low surface tension and a hydrophobic character may promote embolism and inhibit refilling with water in the wilting diseases of larch and spruce caused by Ceratocystis spp.
Acknowledgements
I wish to thank Dr. T. Yamaguchi, Hokkaido Research Center, Forestry and Forest Products Research Institute, and Dr. Y. Yamaoka, Tsukuba University, for providing isolates of two Ceratocystis species.
References
Kuroda, K. 1989. Terpenoids causing tracheid-cavitation in Pinus thunbergii infected by the pine wood nematode (Bursaphelenchus xylophilus). Jpn. J. Phytopathol. 55:170-178.
Kuroda, K. 1991. Mechanism of cavitation development in the pine wilt disease. Eur. J. For. Path. 21:82-89.
Kuroda, K. 1995. Acoustic emission technique for the detection of abnormal cavitation in pine trees infected with pine wilt disease. International symposium on pine wilt disease caused by pine wood nematode (Beijing, China). Proceedings 53-58.
Kuroda, K. 2001. Responses of Quercus sapwood to infection with the pathogenic fungus of a new wilt disease vectored by the barkbeetle Platypus quercivorus. J. Wood Science 47 (in press).
Kuroda, K. & Kuroda, H. 2000. Detection of embolism and acoustic emissions in tracheids under a microscope: Incidence in diseased trees infected with pine wilt, in "New Horizons in Wood Anatomy," ed by Y.S. Kim, Chonnam National Univ. Press Kwangju, Korea, 372-377.
Kuroda, K., Yamada, T., Mineo, K. & Tamura, H. 1988. Effects of cavitation on the development of pine wilt disease caused by Bursaphelenchus xylophilus. Jpn. J. Phytopathol. 54:606-615
Kuroda, K. & Yamada, T. 1996. Discoloration of sapwood and blockage of xylem sap ascent in the trunks of wilting Quercus spp. following attack by Platypus quercivorus. J. Jpn. For. Soc. 78:84-88 (in Japanese with English summary).
Takahata, Y. & Ikeda, T. 2001. Changes of xylem pressure potential in Quercus serrata saplings inoculated with Raffaelea sp., a possible causal fungus of oak mortality in Japan. Proceedings of IUFRO working party 7.02.02 Shoot and Foliage Diseases Meeting (Hyytiala, Finland).